Abstract
Introduction: High-dose methotrexate (HD-MTX) requires extensive monitoring and implementation of supportive care strategies to prevent associated toxicities. Although monitoring protocols for MTX vary across institutions, standardized supportive care measures can improve treatment outcomes. The purpose of this study was to evaluate the impact of a pharmacist-driven monitoring protocol on management of patients with delayed MTX elimination.
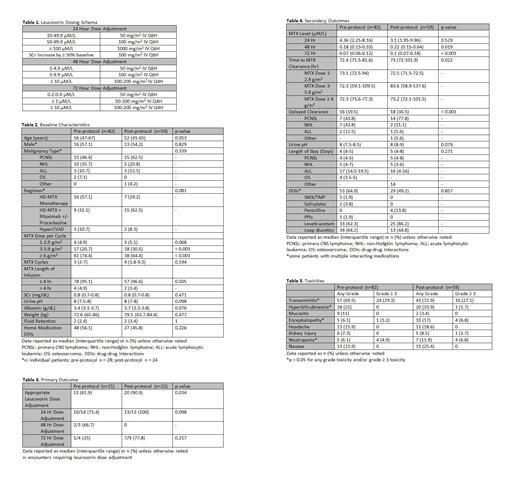
Methods: This was a single-center, retrospective analysis that included hospitalized adult cancer patients receiving HD-MTX therapy (≥ 1 g/m 2). The study consisted of a pre-protocol group (August 2014 to July 2017) and a post-protocol group (August 2017 to August 2020). Patients were included in the analysis more than once if they had a repeat but separate hospital encounter. The primary outcome measure was to compare the rate of appropriate leucovorin dosage adjustments, defined by adherence to institution-specific HD-MTX monitoring guidelines, before and after protocol implementation (Table 1). Secondary outcomes included MTX concentrations at 24, 48, and 72 hours; time to MTX elimination; evidence of delayed MTX clearance; urine pH; length of stay; drug-drug interactions; and incidence of toxicities. Group comparisons for continuous data were made using Mann-Whitney U test and Chi-square or Fisher's exact test was used for categorical data. A p-value of < 0.05 was used to indicate significance.
Results: Of 272 hospital encounters initially identified, 82 pre-protocol encounters and 59 post-protocol encounters were included in the analysis. Thirty-five percent of patient encounters in the pre-protocol group were excluded as a result of inappropriately timed methotrexate levels compared to only 9% in the post-protocol group, indicating improved MTX monitoring after protocol implementation. Baseline characteristics are summarized in Table 2. The most common malignancy was primary CNS lymphoma (53.8%) followed by non-Hodgkin lymphoma (28.8%). HD-MTX monotherapy was the most common regimen used in the pre-protocol group (57.1%) whereas combination therapy with rituximab +/- procarbazine was the most common regimen used in the post-protocol group (62.5%). Twenty-one encounters in the pre-protocol group and 22 encounters in the post-protocol group required leucovorin dose escalations at 24, 48, or 72 hours (Table 3). Following the implementation of the pharmacist-driven protocol, the rate of appropriate leucovorin dose adjustments increased significantly from 61.9% to 90.9% (p = 0.034). The median methotrexate concentration at 48 and 72 hours was higher in the post-protocol group when compared to the pre-protocol group. [0.22 µM/L vs.0.18 µM/L (p = 0.019) and 0.1 µM/L vs. 0.07 µM/L (p < 0.001), respectively]. Median time to methotrexate elimination was slightly longer in patients managed according to the MTX protocol [73 hours vs. 72.4 hours, p = 0.022] with higher rates of delayed clearance noted in these patients [18 (30.5%) vs. 16 (19.5%), p < 0.001]. There were no statistically significant differences in length of stay, urine pH, or drug-drug interactions between the two groups during methotrexate therapy (Table 4). However, a lower percentage of concomitant drug interactions with methotrexate were identified after protocol implementation, suggesting improved drug interaction screening [29 (49.2%) vs. 53 (64.9%), p = 0.857]. Rates of toxicities are shown in Table 5. The post-protocol group experienced a higher incidence of any-grade hyperbilirubinemia [20 (33.9%) vs. 18 (22%), p = 0.019], encephalopathy [10 (17%) vs. 5 (6.1%), p = 0.043], and grade ≥ 3 neutropenia [4 (6.8%) vs. 4 (4.9%), p = 0.009]. These differences in toxicity rates may in part be due to more frequent use of multi-agent chemotherapy in the post-protocol group and greater number of MTX cycles administered to these patients. The incidence of nephrotoxicity was similar in both groups (6 (7.3%) vs. 5 (8.5%), p= 0.493) and in line with previous reports.
Conclusion:The implementation of a pharmacist-driven HD-MTX monitoring protocol improved optimal timing of MTX serum concentrations, resulted in a statistically significant improvement in the rate of appropriate leucovorin dose escalations, and led to enhanced detection of drug interactions. These findings demonstrate the important role pharmacists can play in dosing and monitoring high-risk regimens.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal